Answer:
The answer is D. 0.015%
Step-by-step explanation:
That is the concentration of the Sulfuric Acid solution given in mass per mass percentage (w/w%), it means that the solution has 0.015 grams of Sulfuric Acid per 100 grams of solution. If it is compared with C answer, it has 0.005 grams more. Considering the reaction:
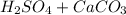 ⇒
⇒
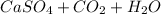
The more
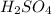 (Sulfuric Acid) is present, the more
(Sulfuric Acid) is present, the more
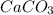 (Limestone) is consumed.
(Limestone) is consumed.