Answer:
PART A 1st order in A and 0th order in B
Part B The reaction rate increases
Step-by-step explanation:
PART A
The rate law of the arbitrary chemical reaction is given by
![-r_A=k*\left[A\right]^\alpha*\left[B\right]^\beta\bigm](https://img.qammunity.org/2020/formulas/chemistry/college/7njhdu3rtpf5op7p7jz563oqe7paf2fqtf.png)
Replacing for the data
Expression 1
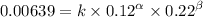
Expression 2
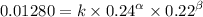
Expression 3
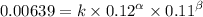
Making the quotient between the fist two expressions

Then the expression for
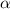
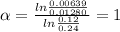
Doing the same between the expressions 1 and 3
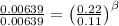
Then
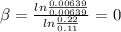
This means that the reaction is 1st order respect to A and 0th order respect to B .
PART B
By the molecular kinetics theory, if an increment in the temperature occurs, the molecules will have greater kinetic energy and, consequently, will move faster. Thus, the possibility of colliding with another molecule increases. These collisions are necessary for the reaction. Therefore, an increase in temperature necessarily produces an increase in the reaction rate.