The slope of the line formed from ln(k) vs 1/T represents
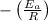
Answer: Option D
Step-by-step explanation:
Based on the Arrhenius equation, we can calculate the activation energies if rate constant is well-known, or vice versa. It also expresses mathematically the relationship: as the activation energy component
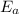 increases, the rate constant k decreases, so the reaction rate decreases.
increases, the rate constant k decreases, so the reaction rate decreases.
The Arrhenius equation is
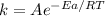
Taking logarithm on both the sides we will have
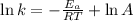
We know the equation y=mx+c where ‘ m ‘ is the slope and c is the Intercept
Equating y=mx+c to the logarithmic equation
we have y= ln k
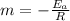
x=1/T and
c =ln A
so, slope
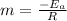 that is the answer
that is the answer