Answer: Option (A) is the correct answer.
Step-by-step explanation:
The given chemical reaction equation is as follows.
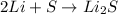
As the number of atoms on both reactant and product side are the same. Hence, it is a balanced chemical reaction equation.
Here, number 2 before lithium atom represents that two atoms of lithium are taking part in the reaction. As there is no coefficient or number placed before the sulfur atom this means that only one atom of sulfur is reacting in the reaction.
Thus, we can conclude that the number 2 present in front of lithium on the reactants side of the equation shows the number of lithium atoms involved in the reaction.