Answer:
Uranium atoms required to be laid side by side to span a distance of 4.96 mm is
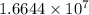 atoms.
atoms.
Step-by-step explanation:
The radius of a uranium atom ,r = 149 pm =
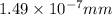
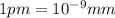
The diameter of a uranium atom ,d =
d = 2r =
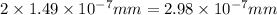
The let the uranium atom to be laid side by side to span a distance of 4.96 mm be x.
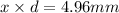
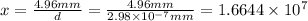 atoms.
atoms.
Uranium atoms required to be laid side by side to span a distance of 4.96 mm is
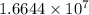 atoms.
atoms.