Answer:
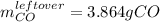
Step-by-step explanation:
Hello,
In this case, the first step is to compute the moles of the reacting both iron (III) oxide and carbon monoxide as follows:
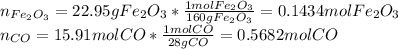
Now, we can take the reacting iron (III) oxide's moles in order to compute the actual consumed carbon monoxide's moles that would completely react with the iron (III) oxide's moles as follows:
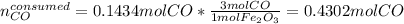
Thus, since there are more available than consumed moles of carbon monoxide, we conclude that the iron (III) oxide is the limiting reagent, in such a way the leftover of carbon monoxide turns out into:
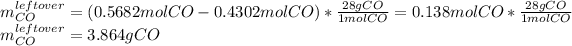
Best regards.