Answer: 2.78 grams
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
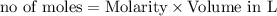
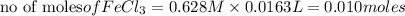
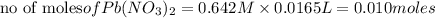
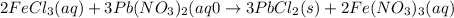
According to stoichiometry:
3 moles of
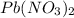 reacts with 2 moles of
reacts with 2 moles of
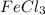
Thus 0.010 moles of
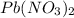 will give=
will give=
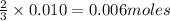 of
of
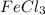
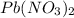 is a limiting reagent as it limits the formation of products and
is a limiting reagent as it limits the formation of products and
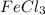 is an excess reagent.
is an excess reagent.
As 3 moles of
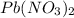 give = 3 moles of
give = 3 moles of
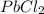
Thus 0.010 moles of
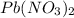 will give=
will give=
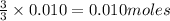 of
of
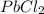
mass of
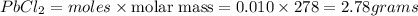
Thus the theoretical yield (in g of precipitate) when 16.3 mL of a 0.628 M solution of iron(III) chloride is combined with 16.5 mL of a 0.642 M solution of lead(II) nitrate is 2.78 grams