Step-by-step explanation:
The given data is as follows.
Current (I) = 3.50 amp, Mass deposited = 100.0 g
Molar mass of Cr = 52 g
It is known that 1 faraday of electricity will deposit 1 mole of chromium. As 1 faraday means 96500 C and 1 mole of Cr means 52 g.
Therefore, 100 g of Cr will be deposited by "z" grams of electricity.
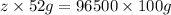
z =
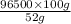
= 185576.9 C
As we know that, Q = I × t
Hence, putting the given values into the above equation as follows.
Q = I × t
185576.9 C =
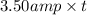
t = 53021.9 sec
Thus, we can conclude that 100 g of Cr will be deposited in 53021.9 sec.