Answer:
Explanation has been given below
Step-by-step explanation:
- Lone pair on N atom in pyridine lies in
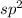 hybridized orbital of N atom.
hybridized orbital of N atom. - Delocalization of this lone pair into requires
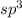 hybridized state of N atom.
hybridized state of N atom.
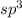 hybridization of N atom is not possible because it will lead to highly strained 6-membered cyclic structure.
hybridization of N atom is not possible because it will lead to highly strained 6-membered cyclic structure.- Hence lone pair on N atom does not involve in delocalization and thereby does not increase electron density in aromatic ring.
- Diagram has been shown below.