Answer:
In this oxidation-reduction reaction oxygen molecule is reduced.
Step-by-step explanation:
Oxidation reaction is defined as the reaction in which an atom looses its electrons.or it can also be understood by saying increase in number of oxygen atoms and decrease in number of hydrogen atom.
Reduction reaction is defined as the reaction in which an atom gains electrons. or it can also be understood by saying decrease in number of oxygen atoms and increase in number of hydrogen atom.
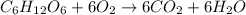
In the given reaction ,carbon in glucose molecule is going under oxidation as number of oxygen atom per carbon atom is increasing.
And oxygen gas molecule is going under reduction as oxygen atom from
 gets attached to two hydrogen atoms.
gets attached to two hydrogen atoms.