Answer:
0.00725 M
Step-by-step explanation:
Considering for
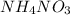
Mass = 5.66 g
Molar mass of
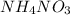 = 80.043 g/mol
= 80.043 g/mol
Moles = Mass taken / Molar mass
So,
Moles = 5.66 / 80.043 moles = 0.0707 moles
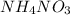 will dissociate as:
will dissociate as:
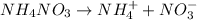
Thus 1 mole of
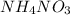 yields 1 mole of ammonium ions. So,
yields 1 mole of ammonium ions. So,
Ammonium ions furnished by
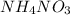 = 1 × 0.0707 moles = 0.0707 moles
= 1 × 0.0707 moles = 0.0707 moles
Considering for
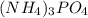
Mass = 4.42 g
Molar mass of
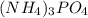 = 149.09 g/mol
= 149.09 g/mol
Moles = Mass taken / Molar mass
So,
Moles = 4.42 / 149.09 moles = 0.0296 moles
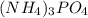 will dissociate as:
will dissociate as:
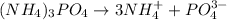
Thus 1 mole of
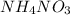 yields 3 moles of ammonium ions. So,
yields 3 moles of ammonium ions. So,
Ammonium ions furnished by
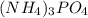 = 3 × 0.0296 moles = 0.0888 moles
= 3 × 0.0296 moles = 0.0888 moles
Total moles of the ammonium ions = 0.0707 + 0.0888 moles = 0.1595 moles
Given that:
Volume = 22.0 L
So, Molarity of the
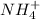 is:
is:
Molarity = Moles / Volume = 0.1595 / 22 M = 0.00725 M