Answer:
3.30%
Step-by-step explanation:
First lets calculate the moles of
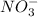 (from the
(from the
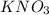 used in the reaction based on the volume and molarity of solution.
used in the reaction based on the volume and molarity of solution.
Moles
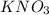 = molarity * volume of
= molarity * volume of
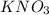
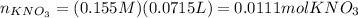
Based on the stoichiometry, we can calculate the moles and mass of Cu.
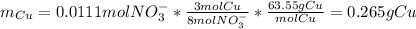
Since we have that 8.00 g of ore contains 0.265 g of Cu, the mass percentage is:
Percentage of Cu in the ore = (0.265 g /8.00 g) x 100% = 3.30%