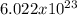Answer:
Average weight=137 pounds=62 kg=
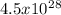 electrons
electrons
Step-by-step explanation:
First, we assume the average weight of an adult human is 137 pounds (62 kg), then we need the percentage of the mass of each element in the human body. Obtaining the molecular weight, and the number of electrons (assuming that your body is electrically neutral) per element using the periodic table. Finally, knowing that Avogadro's number represents units (electrons, atoms, protons) in one mole of any substance; we can estimate the number of electrons in the average human body (You can use your real weight to do the maths) with the equation shown below:
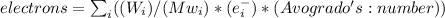
Where:
-
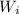 is the weight body multiple by percentage of the mass of each element.
is the weight body multiple by percentage of the mass of each element.
-
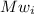 is the molecular weight of each element.
is the molecular weight of each element.
-
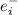 is the number of electrons of each element.
is the number of electrons of each element.
-
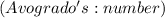 is the Avogadro's number
is the Avogadro's number