Answer:
 for the process is -375 kJ
for the process is -375 kJ
Step-by-step explanation:
- Given reaction is a combination of the two given elementary steps.
- Summation of change in standard enthalpy (
 )of the two elementary reactions give
)of the two elementary reactions give
 of the reaction
of the reaction
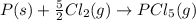 .
.
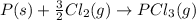 ......
......
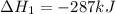
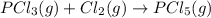 ......
......
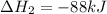
-----------------------------------------------------------------------------------------------------
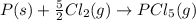
 =
=
