Answer:
new atmospheric pressure is 0.9838 ×
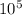 Pa
Pa
Step-by-step explanation:
given data
height = 21.6 mm = 0.0216 m
Normal atmospheric pressure = 1.013 ✕ 10^5 Pa
density of mercury = 13.6 g/cm³
to find out
atmospheric pressure
solution
we find first height of mercury when normal pressure that is
pressure p = ρ×g×h
put here value
1.013 ×
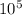 = 13.6 × 10³ × 9.81 × h
= 13.6 × 10³ × 9.81 × h
h = 0.759 m
so change in height Δh = 0.759 - 0.0216
new height H = 0.7374 m
so new pressure = ρ×g×H
put here value
new pressure = 13.6 × 10³ × 9.81 × 0.7374
atmospheric pressure = 98380.9584
so new atmospheric pressure is 0.9838 ×
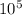 Pa
Pa