Answer : The molar concentration of ethanol in the undiluted cognac is 8.44 M
Explanation :
Using neutralization law,

where,
 = molar concentration of undiluted cognac = ?
= molar concentration of undiluted cognac = ?
 = molar concentration of diluted cognac = 0.0844 M
= molar concentration of diluted cognac = 0.0844 M
 = volume of undiluted cognac = 5.00 mL = 0.005 L
= volume of undiluted cognac = 5.00 mL = 0.005 L
 = volume of diluted cognac = 0.500 L
= volume of diluted cognac = 0.500 L
Now put all the given values in the above law, we get molar concentration of ethanol in the undiluted cognac.
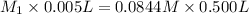
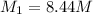
Therefore, the molar concentration of ethanol in the undiluted cognac is 8.44 M