Answer:
4.28 g of KIO3
Step-by-step explanation:
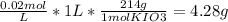
You must use an analytical balance with a precision of 0.1 mg and the measure can be since 4.2372 (0.0198 M) g until 4.3228 (0.0202 M) g to preserve the accurate of 1%. Then dissolve the salt with 100 mL in a beaker with water. Put it into a volumetric flask of 1L and fill with water until the mark.