Answer:
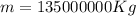
Explanation:
To find the number of kilograms of mercury we need to find how to relate density, mass and, volume. For this we shall recall the density formula:
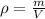
where
 is the density,
is the density,
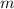 is the mass and,
is the mass and,
 is the volume.
is the volume.
We have the density and want to compute the mass so now we want to know the volume of the pool.
The volume of a rectangular pool is given by the fomula:
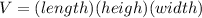 .
.
So for our pool
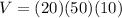 .
.
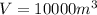 .
.
Our density is in
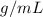 , so the last thing we need to do before computing the mass is to express the density in
, so the last thing we need to do before computing the mass is to express the density in
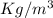 (this is because we want our mass in
(this is because we want our mass in
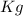 and, we have our volume in
and, we have our volume in
 ).
).
For the density conversion we have to remember that
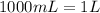
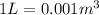
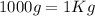
so
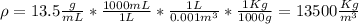 .
.
With this we can finally compute mass:
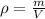
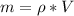
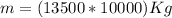
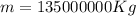 .
.