Answer: The partial pressure of oxygen is 76.56 atm , partial pressure of carbon dioxide is 10.44 atm and the total pressure is 87 atm.
Step-by-step explanation:
According to the ideal gas equation:'

P = Pressure of the gas = ?
V= Volume of the gas =
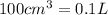
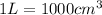
T= Temperature of the gas = 10°C = 373 K
R= Gas constant = 0.0821 atmL/K mol
n= moles of gas= 0.25 +0.034 = 0.284 moles
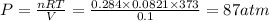
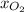 = mole fraction of oxygen=
= mole fraction of oxygen=
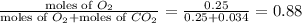 ,
,
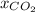 =mole fraction of carbon dioxide=
=mole fraction of carbon dioxide=
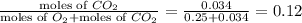
partial pressure of oxygen =
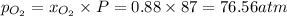
partial pressure of carbon dioxide=
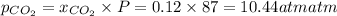
The partial pressure of oxygen is 76.56 atm , partial pressure of carbon dioxide is 10.44 atm and the total pressure is 87 atm.