Answer: The pH of the solution is 9.71
Step-by-step explanation:
1 mole of NaOH produces 1 mole of sodium ions and 1 mole of hydroxide ions.
We are given:
pOH of the solution = 7.2
To calculate the pH of the solution, we need to determine pOH of the solution. To calculate pOh of the solution, we use the equation:
![pOH=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/n477c3o3xy8p6ug3ipfjqh9fd53hdrrjyq.png)
We are given:
![[OH^-]=5.09* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/college/nl0fjakhvqfifxloa319dklszce8ngjad2.png)
Putting values in above equation, we get:
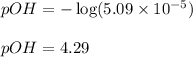
To calculate pH of the solution, we use the equation:
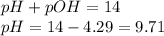
Hence, the pH of the solution is 9.71