Step-by-step explanation:
The given data is as follows.
 = 250 mL,
= 250 mL,
 = 750 mL
= 750 mL
 =
=
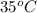 = 35 + 273 K = 308 K
= 35 + 273 K = 308 K
 = 35 + 273 K = 308 K
= 35 + 273 K = 308 K
 = 0.55 atm,
= 0.55 atm,
 = 1.5 atm
= 1.5 atm
P = ? , V = 10.0 L
Since, temperature is constant.
So,
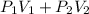 = PV
= PV
Now, putting the given values into the above formula as follows.
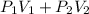 = PV
= PV
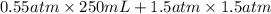 =
=
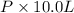
P = 0.126 atm
As, 1 atm = 760 torr. So,
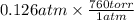 = 95.76 torr.
= 95.76 torr.
Thus, we can conclude that the final pressure, in torr, of the mixture is 95.76 torr.