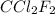Answer: 367 grams
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance weighs equal to the molecular mass and contains avogadro's number
 of particles.
of particles.
Enthalpy of vaporization is the amount of heat released when 1 mole of substance is converted from liquid to gaseous state.
Given : Enthalpy of vapourization of
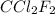 = 17.4 kJ/mol
= 17.4 kJ/mol
To calculate the number of moles, we use the equation:
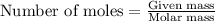
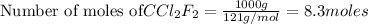
1 mole of
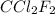 produces heat = 17.4 kJ
produces heat = 17.4 kJ
8.3 moles of
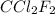 produces heat =
produces heat =
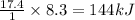
Given : Enthalpy of vapourization of
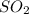 = 6.0 kcal/mol =
= 6.0 kcal/mol =
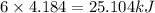 (1kcal=4.184kJ)
(1kcal=4.184kJ)
25.104 kJ heat is produced by = 1 mole of
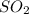
144 kJ heat is produced by =
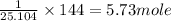 of
of
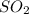
Mass of
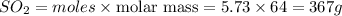
Thus 367 grams of
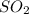 must be evoparated o remove as much heat as evaporation of 1.00 kg of
must be evoparated o remove as much heat as evaporation of 1.00 kg of