Answer:
The mass percent of copper as element is the same.
Step-by-step explanation:
First of all we need the reaction that is presented below:
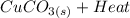 →
→
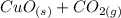
The mass percent of copper (Cu) as element is the same because of during the reaction the element only transform its nature from copper carbonate (
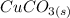 ) to copper oxide (
) to copper oxide (
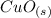 ), the latter is a solid and will remain in the system.
), the latter is a solid and will remain in the system.
On the other hand, you will note that the global percentage mass will be small because of the reaction produce (
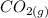 ) that is a gas and this one will escape for the system.
) that is a gas and this one will escape for the system.
Have a great day!