Answer: The sample that contains the greater number of molecules is water.
Explanation: To calculate the number of molecules, it is used the Avogadro's number (
 particles/molecules). So, considering that the mass to water and carbon dioxide is 1g (it can be any other number), the relationship between moles and molar weight is:
particles/molecules). So, considering that the mass to water and carbon dioxide is 1g (it can be any other number), the relationship between moles and molar weight is:
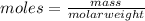
To water:
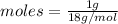 = 0.05 moles.
= 0.05 moles.
Therefore,
1 mol ----
 molecules
molecules
0.05 moles ---- x
x =
 molecules of water.
molecules of water.
To carbon dioxide:
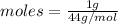 = 0.02 moles.
= 0.02 moles.
Therefore,
1 mol ----
 molecules
molecules
0.02 moles ---- y
y =
 molecules of carbon dioxide.
molecules of carbon dioxide.