Answer : The mass of oxygen formed must be 3.8 grams.
Explanation :
Law of conservation of mass : It states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
This also means that total mass on the reactant side must be equal to the total mass on the product side.
The balanced chemical reaction will be,
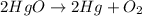
According to the law of conservation of mass,
Total mass of reactant side = Total mass of product side
Total mass of
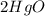 = Total mass of
= Total mass of
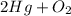
As we are given :
The mass of
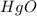 = 25.3 grams
= 25.3 grams
The mass of
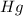 = 23.4 grams
= 23.4 grams
So,
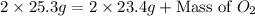
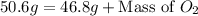
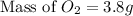
Therefore, the mass of oxygen formed must be 3.8 grams.