Answer:
1:1
Step-by-step explanation:
NaCl is formed when sodium ion
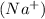 combine with chloride ion
combine with chloride ion
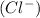
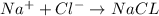
In this
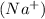 is a cation which is formed by losing an electron from sodium element .Sodium loses one electron to become stable because it has one extra valence electron
is a cation which is formed by losing an electron from sodium element .Sodium loses one electron to become stable because it has one extra valence electron
And
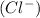 is a anion which is formed by gaining one electron to become stable
is a anion which is formed by gaining one electron to become stable
So in NaCl there is 1 sodium and and 1 chloride ion so ratio will be 1:1