Answer:
141.7475 milliliters of water must be added.
Step-by-step explanation:
Heat gained by water will be equal to heat loss by the coffee solution.
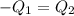
Mass of water =
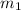
Specific heat capacity of water=
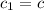
Initial temperature of the water=
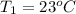
Final temperature of water coffee solution=
 = 60 °C
= 60 °C
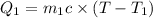
Mass of coffee solution=
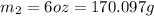
1 oz = 28.3495 grams
Specific heat capacity of coffee=
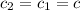
Initial temperature of the coffee solution=
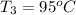
Final temperature of water coffee solution=
 =T=60 °C
=T=60 °C
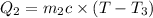
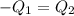
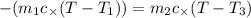
On substituting all values:
we get,
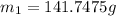
Density of the water = 1.00 g/mL
Volume of the water =
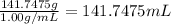
141.7475 milliliters of water must be added.