Answer and Explanation:
Difference between heat capacity and specific heat ;
- Heat capacity is the heat required to raise the temperature of any substance by 1° Celsius whereas the specific heat is the heat required to raise the temperature of unit mass of the substance by 1°C
- Heat capacity depends on the mass of the substance
- Heat capacity of any substance is given by
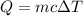 where m is mass c is specific heat and
where m is mass c is specific heat and
 is the change in temperature
is the change in temperature