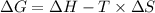Answer:
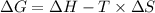
Step-by-step explanation:
The Gibbs free energy in thermodynamics is a potential which is used to calculate maximum of the reversible work which is performed by a specific thermodynamic system at constant temperature (isothermal) as well as pressure (isobaric).
The expression for the change in free energy is: