Answer:
The pH of the new HCl solution is 0.53.
Step-by-step explanation:
Volume of the HCl solution
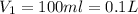
Molarity of HCl solution ,
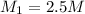
Volume of water added = 100 mL = 0.1 L
Molarity of HCl after addition of 0.75 L of water =

Final volume of the HCl solution after addition of 0.75 L of water =

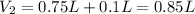
 [
[
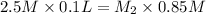
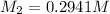
HCl is a monotonic acid and its one mole will give 1 mole of hydrogen ions in aqueous solution.
Molarity of hydrogen ion =
![[H^+]=0.2941 M](https://img.qammunity.org/2020/formulas/chemistry/college/r4fremaxnus06m75bvx4b2el83zz5834aq.png)
![pH=-\log [H^+]=-\log [0.2941 M]=0.53](https://img.qammunity.org/2020/formulas/chemistry/college/dtk4u5h73eavjhawv0jfcyqicimx1qpylr.png)
The pH of the new HCl solution is 0.53.