Answer:
1) The equilibrium constant for the required reaction is
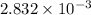 .
.
2) 1.2474 M the concentration of ammonia needed to form 0.060 M of complex.Explanation:
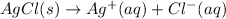
Solubility product of silver chloride:
![K_(sp)=1.77* 10^(-10)=[Ag^+][Cl^-]](https://img.qammunity.org/2020/formulas/chemistry/college/ugj94o73cdcvsp4ljh9xr8y2a0jggvrca2.png) ..(1)
..(1)
Formation constant of
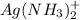 :
:
![K_f=1.6* 10^7=([Ag(NH_3)_2^(+)])/([Ag^+][NH_3]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/j2fq6gris1pdet415n3qz7swhmbfih850k.png) ..(2)
..(2)
Reactions solid silver chloride and liquid ammonia:
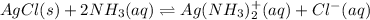
Expression of an equilibrium constant of the above reaction can be written as:
![K=([Ag(NH_3)_2^(+)][Cl^-])/([AgCl][NH_3]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/aoatx3ttvc5evwqjzcjctn4d4wgmkrrrsu.png)
[AgCl] = solid = 1
![K=([Ag(NH_3)_2^(+)][Cl^-])/([1][NH_3]^2)* ([Ag^+])/([Ag^+])](https://img.qammunity.org/2020/formulas/chemistry/college/qu347tl1wfeqnj66fqjjekod5g4vhiw57w.png)
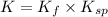 (from 1 and 2)
(from 1 and 2)

The equilibrium constant for the required reaction is
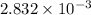 .
.
2)
Concentration of complex at equilibrium :
![[Ag(NH_3)_2^(+)]](https://img.qammunity.org/2020/formulas/chemistry/college/dt82pvm4ckk656v1exeabr94upqfqty5sm.png) = 0.060 M
= 0.060 M
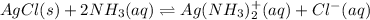
Initaly
x 0 0
At equilibrium
x- 2(0.060) 0.060 0.060
![K=([Ag(NH_3)_2^(+)][Cl^-])/([1][NH_3]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/2dn83ftkwoo5hg6e2ifx4tueu7quxcipgo.png)
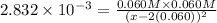
x = 1.2474 M
1.2474 M the concentration of ammonia needed to form 0.060 M of complex.