Answer:
The correct answer is "Higher", hence the statement becomes The lower the pH number the higher the hydrogen ion concentration.
Step-by-step explanation:
By the definition of pH we have
![pH=-log[H^(+)]](https://img.qammunity.org/2020/formulas/medicine/college/11uazg5asji4j9glisfdrlkgsy6l390iy3.png)
where
![[H^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/axnvi9gez4h3rovfp1qidd16ya8d7gzbon.png) is the concentration of hydrogen ions in the solution in moles/Liter
is the concentration of hydrogen ions in the solution in moles/Liter
Thus the concentration of
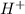 ions in a solution is obtained by taking anti-log on both sides of the equation above thus we have
ions in a solution is obtained by taking anti-log on both sides of the equation above thus we have
![[H^(+)]=antilog(-pH)\\\\\therefore [H^(+)]=10^(-pH)\\\\\therefore [H^(+)]=(1)/(10^(pH))](https://img.qammunity.org/2020/formulas/medicine/college/bwnttbb9ztfq9uyeprgn8uel6p6q9p5kyh.png)
Thus we can observe lower the pH of a solution higher is the hydrogen ion concentration in the solution since the 2 are inversely related.