Answer:
at the oxygen end electron density lies
Step-by-step explanation:
Carbonyl group is a functional group which is formed when oxygen combined with carbon through a double bond
So the carbonyl group is
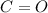 in this the oxygen has more electronegativity than carbon so oxygen attracts the electron density towards itself
in this the oxygen has more electronegativity than carbon so oxygen attracts the electron density towards itself
So the electron density lies on the oxygen in a carbonyl group