Answer: Option (d) is the correct answer.
Step-by-step explanation:
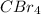 has a tetrahedral structure and since, bromine is more electronegative than carbon atom. So, each bromine atom will pull the electrons more towards itself.
has a tetrahedral structure and since, bromine is more electronegative than carbon atom. So, each bromine atom will pull the electrons more towards itself.
As a result, dipole moment will cancel out each other. Therefore,
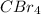 molecule will be non-polar in nature.
molecule will be non-polar in nature.
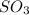 has trigonal planar geometry and oxygen atom being more electronegative in nature than sulfur atom will attract the electrons more towards itself.
has trigonal planar geometry and oxygen atom being more electronegative in nature than sulfur atom will attract the electrons more towards itself.
Hence, dipole moment will be cancelled out. Therefore,
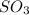 molecule is non-polar in nature.
molecule is non-polar in nature.
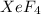 has square planar geometry and fluorine atom being more electronegative in nature than xenon atom will attract the electrons more towards itself.
has square planar geometry and fluorine atom being more electronegative in nature than xenon atom will attract the electrons more towards itself.
Hence, dipole moment will be cancelled out. Therefore,
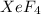 molecule is non-polar in nature.
molecule is non-polar in nature.
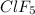 has square pyramidal geometry and fluorine atom being more electronegative in nature than xenon atom will attract the electrons more towards itself.
has square pyramidal geometry and fluorine atom being more electronegative in nature than xenon atom will attract the electrons more towards itself.
There will be an unpaired dipole moment that doesn't get cancelled out. Hence,
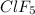 molecule will be polar in nature.
molecule will be polar in nature.
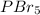 has trigonal bipyramidal geometry and bromine atom being more electronegative in nature than phosphorous atom will attract the electrons more towards itself.
has trigonal bipyramidal geometry and bromine atom being more electronegative in nature than phosphorous atom will attract the electrons more towards itself.
Hence, dipole moment will be cancelled out. Therefore,
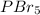 molecule is non-polar in nature.
molecule is non-polar in nature.
Thus, we can conclude that out of the given options
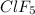 is polar in nature.
is polar in nature.