Answer:
The entropy change of the system is 0.1265 J/K.
Step-by-step explanation:
Given that,
Current = 0.5 A
Resistor = 2 Ω
Time = 100 s
Temperature = 300 K
Heat capacity = 0.24 J/K
We need to calculate the heat produced
Using formula of heat
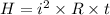
Where, i = current
R = resistor
t = time
Put the value into the formula
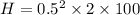
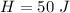
We need to calculate the change in temperature
Using formula of temperature
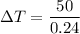
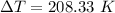
Now, The temperature is
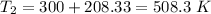
We need to calculate the entropy change of the system
Using formula of entropy
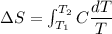
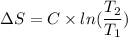
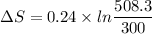
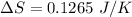
Hence, The entropy change of the system is 0.1265 J/K.