Answer:
Explanation has been given below
Step-by-step explanation:
- Rate law =
![k[benzaldehyde][CN^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/p67zca202p5mbu22l9lpbmis1yuw2pye40.png) , where k is rate constant and species inside third bracket represents concentrations.
, where k is rate constant and species inside third bracket represents concentrations. - Clearly, rate depends upon concentration of free
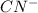
- In acidic medium (pH = 5.6), concentration of free
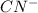 is negligible as HCN is a weak acid. So, reaction between benzaldehyde and HCN does not occur at all.
is negligible as HCN is a weak acid. So, reaction between benzaldehyde and HCN does not occur at all. - In neutral medium (pH = 7), concentration of cyanide ion somewhat increases. Therefore reaction is slow in neutral pH
- In basic medium (pH > 7), concentration of cyanide ions become sufficient to make the reaction spontaneous. In basic condition, concentration of cyanide increases due to increase in ionization of HCN
- Full reaction mechanism has been shown below.