Answer: The new volume at STP is 1125.43 mL.
Step-by-step explanation:
STP conditions:
Pressure of the gas is 760 mmHg and temperature of the gas is 273 K
To calculate the volume when temperature and pressure are changed, we use the equation given by combined gas law. The equation follows:
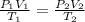
where,
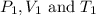 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas
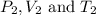 are the final pressure, volume and temperature of the gas
are the final pressure, volume and temperature of the gas
We are given:
Conversion factor: 1 cm = 10 mm
![P_1=175cmHg=1750mmHg\\V_1=555mL\\T_1=37^oC=[37+273]=310K\\P_2=760mmHg\\V_2=?mL\\T_2=273K](https://img.qammunity.org/2020/formulas/chemistry/college/kouobdrb2nk8zrbhewoh8w4ehhoypdp19b.png)
Putting values in above equation, we get:
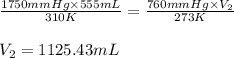
Hence, the new volume at STP is 1125.43 mL.