Answer: The outermost valence electron enters the p orbital.
Step-by-step explanation:
Valence electrons are defined as the electrons which are present in outer most orbital of an atom.
Sulfur is the 16th element of the periodic table having 16 electrons.
Electronic configuration of sulfur atom is
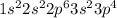
The number of valence electrons are 2 + 4 = 6
These 6 electrons enter s-orbital and p-orbital but the outermost valence electron will enter the p-orbital.
Hence, the outermost valence electron enters p orbital.