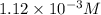Answer : The
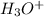 ion concentration is,
ion concentration is,
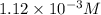 and the pH of a buffer is, 2.95
and the pH of a buffer is, 2.95
Explanation : Given,
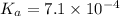
Concentration of
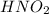 (weak acid)= 0.26 M
(weak acid)= 0.26 M
Concentration of
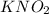 (conjugate base or salt)= 0.89 M
(conjugate base or salt)= 0.89 M
First we have to calculate the value of
 .
.
The expression used for the calculation of
 is,
is,
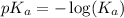
Now put the value of
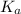 in this expression, we get:
in this expression, we get:
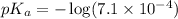
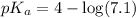
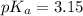
Now we have to calculate the pH of the solution.
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2020/formulas/chemistry/college/6wyuhr9b7n0qwlgrnwgg688yylfbvv3wby.png)
![pH=pK_a+\log ([KNO_2])/([HNO_2])](https://img.qammunity.org/2020/formulas/chemistry/college/22ds95bif6pi392lz1jntraghctctwbvwc.png)
Now put all the given values in this expression, we get:
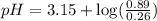
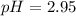
The pH of a buffer is, 2.95
Now we have to calculate the
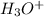 ion concentration.
ion concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xj6fwrpeduepfcp6k4cv1s827uf5c0pbp1.png)
![2.95=-\log [H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/cs2bvzv2xxi54lrdvndkcmfd206jcw414a.png)
![[H_3O^+]=1.12* 10^(-3)M](https://img.qammunity.org/2020/formulas/chemistry/college/4gakqg2cfydmaybeuq028myvlc7k8kbxob.png)
The
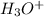 ion concentration is,
ion concentration is,