Answer:
8
Step-by-step explanation:
Ammonium ion
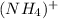 is formed when ammonia
is formed when ammonia
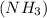 reacts with hydrogen ion
reacts with hydrogen ion

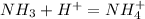
In
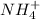 the nitrogen has 5 valence electron and there are 4 hydrogen each has 1 valence electron
the nitrogen has 5 valence electron and there are 4 hydrogen each has 1 valence electron
So total number of valence electron =4+5=9
But
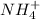 has 1 positive charge on it, it means it losses 1 electron
has 1 positive charge on it, it means it losses 1 electron
So total number of valence electron in poly atomic ion of
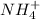 is 9-1=8
is 9-1=8