Answer:
Reaction 2 is 2.333 times faster than reaction 1.
Step-by-step explanation:
The reaction rate is measured considering the concentration of reactant used or product made and the time interval. This can be expressed in the following equation:
![rate = ([reactant])/(Δtime)](https://img.qammunity.org/2020/formulas/chemistry/high-school/g4lz4jxcayr62h72dfg96awaommhqnw149.png)
Therefore, the higher the concentration of reactant used, the faster the reaction will be. If we consider that both Reaction 1 and Reaction 2 happen during the same time interval (Δt), we can express their rates:
![R1 = ([0.240 mol/L])/(Δt) \\R2 = ([0.560 mol/L)/(Δt)](https://img.qammunity.org/2020/formulas/chemistry/high-school/8zn9ja36is3y12udrlzq5ammfyjw9znbg0.png)
Dividing R2 per R1:
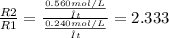
Thus, Reaction 2 is 2.333 times faster than Reaction 1.