Step-by-step explanation:
It is known that when acid is stored in a metal container then there occurs a chemical reaction between them.
During this chemical reaction there will be formation of a metal salt along with liberation of hydrogen gas.
For example, when HCl is stored in a zinc container then the chemical reaction which takes place is as follows.
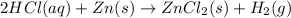
So, in order to avoid this type of chemical reaction we do not store acids in metal containers.