Answer: The pH of weak acid is 1.001
Step-by-step explanation:
We are given:
Concentration of HF = 0.1 M
The chemical equation for the dissociation of HF follows:
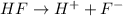
Initial: 0.1
At eqllm: 0.1-x x x
The equation for equilibrium constant follows:
![K_a=([H^+][F^-])/([HF])](https://img.qammunity.org/2020/formulas/chemistry/college/cyikt0fpaa32yo78y0yirzzb8qyq4aoyhb.png)
We are given:
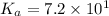
Putting values in above equation, we get:
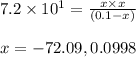
Neglecting the negative value of 'x' because concentration cannot be negative
So, concentration of
 = 0.0998 M
= 0.0998 M
To calculate the pH of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
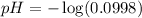
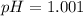
Hence, the pH of weak acid is 1.001