Answer:the pH is 12
Step-by-step explanation:
First We need to understand the structure of trimethylamine
Due to the grades of the bond in the nitrogen with a hybridization sp3 is 108° approximately, then is generated a dipole magnetic at the upper side of the nitrogen, this dipole magnetic going to attract a hydrogen molecule of the water making the water more alkaline
C3H9N+ H2O --> C3H9NH + OH-
![k=([C3H9NH]*[OH-])/([C3H9N])](https://img.qammunity.org/2020/formulas/chemistry/college/l0y8gqr3yjvzq6agrr31tjjhe3ve13kz36.png)
Then:
The concentration of the trimethylamine is 0.3 and the concentration of the ion C3H9NH is equal to the OH- relying on the stoichiometric equation. We could find the concentration of the OH- ion with the square root of the multiplication between k and the concentration of trimethylamine
[OH-]=
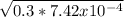
[OH-]=0.01
pH=14-(-log[OH-])
pH=12