Answer:
bond angle = 120 °
Step-by-step explanation:
Lewis structure is used to represent the arrangement of the electrons around the atom in a molecule. The electrons are shown by dots and the bonding electrons are shown by line between the two atoms.
For
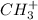 ,
,
The number of valence electrons =
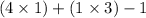 = 6
= 6
The skeleton structure of the compound is in which one C molecule act as a central atom and is bonded to the three H with one sigma bond each.
Number of valence electrons used in skeletal structure = 6
So,
Remaining valence electrons = 0
The Lewis structure of the compound is shown below:
H
:
C⁺
: :
H H
The geometry is trigonal planar which has an angle of 120 °.