Answer:
10°C
Step-by-step explanation:
Heat gain by water = Heat lost by the slice of pizza
Thus,
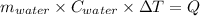
For water:
Volume = 50.0 L
Density of water= 1 kg/L
So, mass of the water:
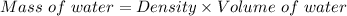
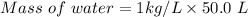
Mass of water = 50 kg
Specific heat of water = 1 kcal/kg°C
ΔT = ?
For slice of pizza:
Q = 500 kcal
So,

ΔT = 10°C
Increase in temperature = 10°C