Answer:
Sucrose 342.34
Mysristic acid 228.42
Step-by-step explanation:
The first step is to write out the formula of the substance in question. Let us start with sucrose.
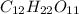 . Then use that to help you determine the molecular weights using the relative atomic mass of each species.
. Then use that to help you determine the molecular weights using the relative atomic mass of each species.
molecular weight = (12.01*12) + (1.01*22) + (16.00*11) = 342.34
Be sure to write no units for this answer. This is not the molar mass, this is the relative molecular mass. It's on a relative scale not in grams
We repeat the steps above for mysristic acid, formula
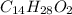 .
.
molecular weight = (12.01*14) + (1.01*28) + (16*11) = 228.42