Step-by-step explanation:
Relation between
 and
and
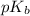 is as follows.
is as follows.
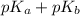 = 14
= 14
Hence, putting the given values of
 in order to calculate the value of
in order to calculate the value of
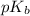 as follows.
as follows.
For
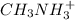 :
:
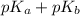 = 14
= 14
10.6 +
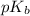 = 14
= 14
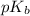 = 14 - 10.6
= 14 - 10.6
= 3.4
For
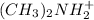 :
:
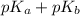 = 14
= 14
10.7 +
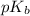 = 14
= 14
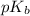 = 14 - 10.7
= 14 - 10.7
= 3.3
Hence, smaller is the value of
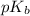 of a specie more will be the value of its
of a specie more will be the value of its
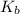 . Therefore, stronger will be the base.
. Therefore, stronger will be the base.
Thus, we can conclude that
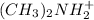 will be the stronger base.
will be the stronger base.