Answer:
number of neutrons an
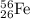 nucleus must capture equal to 17
nucleus must capture equal to 17
Step-by-step explanation:
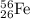 has mass number of 56 and atomic number of 26
has mass number of 56 and atomic number of 26
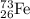 has mass number of 73 and atomic number of 26
has mass number of 73 and atomic number of 26
Neutron (
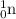 has a mass number of 1 and atomic number of 0.
has a mass number of 1 and atomic number of 0.
Mass number and atomic number in both side of reaction should be equal.
As atomic number does not change during neutron capture therefore number of neutrons required for the given transformation will be equal to change in mass number of Fe.
So, number of neutrons an
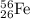 nucleus must capture = (73-56) = 17
nucleus must capture = (73-56) = 17