Answer:
The possible quantum numbers for Ca atom when it is in excited
n = 4
l= 1
m =-1,0,1
Step-by-step explanation:
Given that,
Atomic number = 20
The configuration of Ca
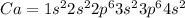
So, The principle quantum number is n =4.
The azimuthal quantum number can take value from 0,1,2...(n-1)
In the ground state, l= 0
When it is excited to p state
Then, l = 1
The magnetic quantum number can take value from l to -l
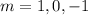
Hence, The possible quantum numbers for Ca atom when it is in excited
n = 4
l= 1
m =-1,0,1